Rust & corrosion kit
Click Image for Gallery
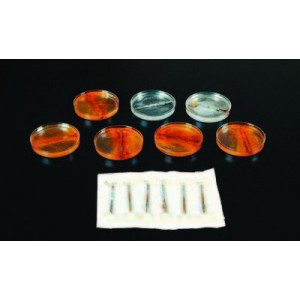
This visually striking lab clearly illustrates the processes of rusting, corrosion and cathode inhibition. Iron metal will rust in the presence of air and water, a process that accelerates in ocean water. Yet, in the presence of another metal (such as magnesium) the nails will not rust for a very long time as the magnesium sacrifices itself by supplying electrons to the iron nail. Eventually the magnesium will vanish, and a white solid will form on the bottom of the petri dish. Developed by Ray Steigvilas. Includes materials for five student groups and instructions. Grades 7–12.